Respire
Devoted to Breath

Respire : Pioneering DPI Manufacturing in Nepal
In the ever-evolving landscape of pharmaceuticals, innovation and progress continue to drive advancements in healthcare. Nepal, a nation mostly known only for its rich cultural heritage and breathtaking landscapes, is now stepping onto the global pharmaceutical stage with a groundbreaking achievement. The introduction of M.D.H Pharmaceutical’s Respire Division, Nepal's very first DPI (Dry Powder Inhaler) producing facility, heralds a new era of pharmaceutical excellence in the country.
Respire : Our Story
Respire emerged as a testament to the power of vision and the spirit of innovation in Nepal . Our story is one of determination, commitment, and the relentless pursuit of excellence in the realm of respiratory health, setting the stage for a transformative journey that began with a wish for breath for all and with that began our dedication to Inhalation Remedies.
Empowering Lives, One Breath at a Time: Respire is dedicated to empowering lives by prioritizing respiratory health. Our DPIs provide a breath of fresh air for individuals managing conditions such as asthma and chronic obstructive pulmonary disease (COPD), offering a reliable and accessible solution for improved respiratory well-being.

CHARTING SUCESS TOGETHER
Respire Division of M.D.H Pharmaceuticals is proud to announce our partnership with leading global companies to deliver unparalleled products for your health and well-being.
By joining forces with industry giants such as ACG Capsules, Copley and Shimadzu , we are committed to providing you with the ultimate solutions for respiratory ailments.
ACG Capsules, renowned for their expertise in pharmaceutical packaging solutions, ensures that our products are delivered to you with the highest standards of quality and safety. With their innovative capsule technology, we guarantee optimal delivery and efficacy of our respiratory medications.
Copley's next-generation impacter sets a new standard in inhaler testing, allowing us to precisely measure the performance of our inhalation products. With this cutting-edge technology, we can ensure that each dose of medication is delivered efficiently and accurately to your lungs, providing you with the relief you need. Additionally, our partnership with Shimadzu brings the latest in HPLC technology to our manufacturing process.
The Shimadzu Nexera HPLC system enables us to rigorously test and analyze the quality of our products, ensuring purity, potency, and consistency with unmatched precision.
We are proud to leverage our state-of-the-art Class 10,000 cleanroom facility to manufacture our dry powder inhaler products. With meticulous attention to detail and stringent quality control measures, our cleanroom provides the optimal environment for producing inhalers of exceptional quality and reliability.
At Respire Division, we are dedicated to breath and advancing respiratory health through innovation, collaboration, and excellence. With our world-class partners by our side, we are committed to delivering superior products that empower you to breathe easier and live healthier lives. Experience the difference with Respire Division of M.D.H Pharmaceuticals – where quality meets innovation for your respiratory needs.

Our Products
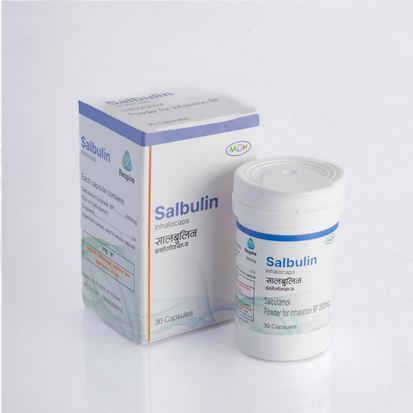
Salbulin
Salbutamol Sulphate 200 mcg powder for Inhalation IP

Foraflo 250 foraflo 500
Formeterol Fumarate Dihydrate 6mcg
Fluticasone Propionate250/500 Powder for Inhalation IP
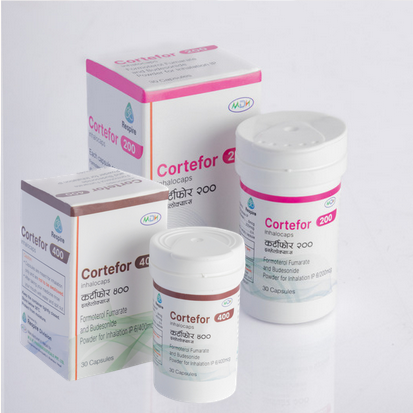
Cortefor 400 cortefor 200
Formoterol Fumarate 6mcg and Budesonide Powder200/400 for Inhalation IP
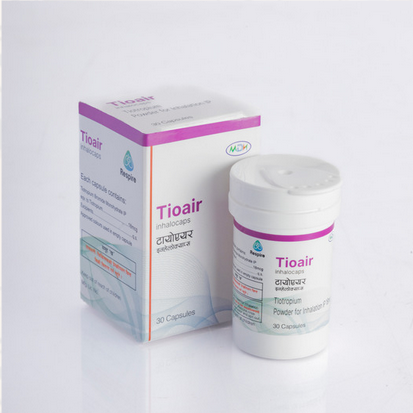
tioair
Tiotropium powder 18 mcg for inhalation
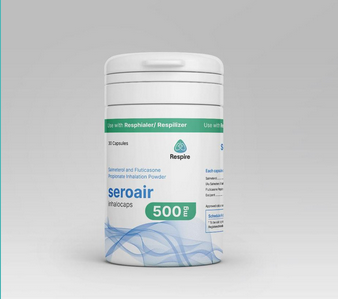
seroair 250 seroair 500
Salmeterol 50mcg and Fluticasone Propionate Powder for Inhalation IP

REspirer
Inhalation Device for Inhalocaps

Cortefor-g
Glycopyrrolium as Glycopyrrolate IP (25mcg) Formoterol Fumarate (12mcg)
Budesonide IP (400mcg)

foraglyde
Glycopyrrolium as Glycopyrrolate IP
25mcg Formoterol Fumarate 12mcg For Inhalation
Our Products